Have you ever picked up a prescription and noticed the pill looks exactly like your brand-name drug-but the box says something totally different? Maybe it’s labeled with just the chemical name, no fancy logo, and costs less. You might wonder: is this a fake? A cheaper version? Or something else entirely? This is where authorized generics come in-and they’re one of the most misunderstood parts of the drug market.
What Exactly Is an Authorized Generic?
An authorized generic is the exact same medication as the brand-name drug you know, but sold without the brand name on the label. The FDA defines it clearly: it’s an approved brand-name drug that’s marketed under its generic name. Everything else? Identical. Same active ingredient. Same inactive ingredients. Same shape, size, and how it’s made. The only difference? No brand name on the packaging. This isn’t a copy. It’s not a lookalike. It’s the real thing-produced by the same company that made the brand-name version. For example, if you take Concerta for ADHD, the authorized generic is made by the same manufacturer, using the same factory, same formula, same quality control. It just doesn’t say "Concerta" on it.How Is It Different From a Regular Generic?
Regular generics are approved through a process called the Abbreviated New Drug Application (ANDA). That means another company makes a version of the drug and proves it works the same way as the brand. They don’t have to repeat all the original clinical trials-they just show bioequivalence. But here’s the catch: they can change the inactive ingredients. That’s things like fillers, dyes, or binders. For most people, that doesn’t matter. But for some, those small changes can cause issues-like stomach upset, allergic reactions, or just feeling like the drug doesn’t work as well. Authorized generics skip that whole process. They don’t need ANDA approval because they’re already covered under the original brand’s New Drug Application (NDA). The manufacturer doesn’t have to prove anything new. They just notify the FDA and start selling. And because they’re made from the exact same recipe, they don’t have any of the inactive ingredient differences you might find in traditional generics.Who Makes Authorized Generics?
There are two ways this works:- The brand-name company makes the authorized generic themselves and sells it under a different label.
- The brand-name company licenses the formula to another company to make and sell it.
- Colcrys (brand) → Colchicine (authorized generic)
- Celebrex (brand) → Celecoxib (authorized generic)
- Unithroid (brand) → Levothyroxine (authorized generic)
Why Do Drug Companies Make Them?
At first glance, this seems strange. Why would a company that just spent billions developing a drug turn around and sell a cheaper version of it? The answer is business strategy. When a brand-name drug’s patent expires, other companies can make generics. But that usually means the brand’s sales drop fast-sometimes by 80% or more. To protect their market share, brand manufacturers started launching authorized generics just before or right after the first generic hits the market. Here’s how it plays out:- Brand drug’s patent expires.
- First generic manufacturer gets 180 days of exclusivity (thanks to the Hatch-Waxman Act).
- Brand company launches its own authorized generic-same drug, lower price.
Are Authorized Generics Cheaper?
Sometimes. Usually. But not always. Right after launch, authorized generics are often priced lower than the brand-sometimes 20-30% cheaper. That’s a real savings. But because they’re made by the brand company, they’re often more expensive than traditional generics that come later, especially when multiple generic makers enter the market. Think of it like this: the first generic might be $10. The authorized generic might be $15. The brand is $50. You save $35 by switching to the authorized generic. But if a second generic comes in at $8, you save even more. The real value isn’t always in the lowest price-it’s in consistency. If you’ve had bad reactions to other generics, or just feel better on your brand, the authorized generic gives you the same effect without the brand-name cost.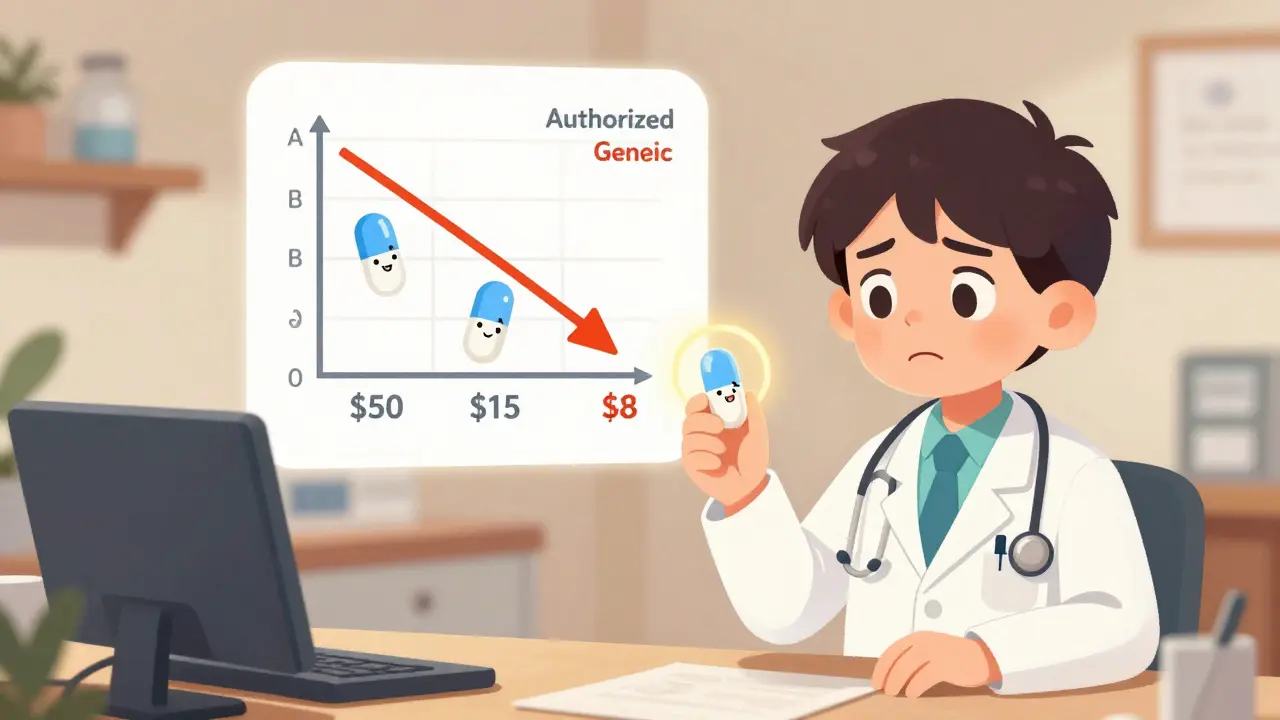
 Jan, 12 2026
Jan, 12 2026

Lance Nickie
January 13, 2026 AT 19:50authrozed generics? more like authorized ripoffs lmao
Rosalee Vanness
January 14, 2026 AT 02:59Okay, I’ve been on levothyroxine for 12 years and I swear, every time my pharmacy switches me to a new generic, I feel like I’ve been hit by a bus for three days-fatigue, brain fog, the whole deal. Then I asked for the authorized generic-Unithroid’s version-and it was like someone turned my internal dimmer switch back to full brightness. No joke. Same pill, same factory, same everything. The only difference? The box says ‘levothyroxine sodium’ instead of ‘Unithroid.’ I don’t care what it’s called, I care that my thyroid isn’t staging a rebellion every month. Seriously, if you’ve ever felt ‘off’ on a generic, don’t assume it’s all in your head. Sometimes it’s the fillers. Sometimes it’s the dye. Sometimes it’s just the fact that your body remembers what it’s used to. Ask for the authorized version. It’s not magic. It’s just science that doesn’t mess around.
Lethabo Phalafala
January 15, 2026 AT 05:50THIS IS WHY I HATE PHARMACEUTICAL COMPANIES. THEY MAKE BILLIONS ON A DRUG, THEN LAUNCH THEIR OWN ‘CHEAPER’ VERSION TO STEAL BACK THEIR OWN CUSTOMERS LIKE IT’S A GAME OF CHESS AND WE’RE THE PAWNS. I’M NOT A FOOL. I KNOW THIS ISN’T CHARITY. IT’S A BUSINESS MOVE SO THEY DON’T HAVE TO WATCH THEIR PROFITS CRUMBLE. BUT STILL-IF IT WORKS AND IT’S SAFE? I’LL TAKE IT. JUST DON’T ACT LIKE YOU’RE MY SAVIOR, PFIZER. I KNOW WHAT YOU’RE DOING.
Priyanka Kumari
January 16, 2026 AT 03:18As someone from India where generic drugs are the lifeline for millions, I find this fascinating. In our system, generics are often the only option-and they’re life-saving. But hearing that even in the U.S., where brand drugs dominate, there’s this middle ground-authentic, identical, but affordable-it gives me hope. It’s not about cheapness. It’s about access with integrity. Thank you for explaining this so clearly. Maybe one day, this model can inspire better systems elsewhere too.
Damario Brown
January 17, 2026 AT 17:36so the brand company makes a generic of their own drug to undercut other generics?? wow what a shocker. next they'll invent water that's not wet. also i bet they use the same fillers that cause the 'bad reactions' in other generics but just rename them 'proprietary excipients' and charge $20 extra. this isn't transparency. it's brand manipulation wrapped in a lab coat. also the FDA doesn't regulate inactive ingredients? really? that's why people die from lactose intolerance in pills. #pharmafraud
Milla Masliy
January 18, 2026 AT 07:15I love how this post doesn’t just explain the science but also the quiet, systemic drama behind it. It’s not just medicine-it’s capitalism in pill form. The fact that companies can legally make the exact same thing and sell it under a different label just to keep profits from collapsing… it’s brilliant. And kind of sad. I’ve had patients cry because they couldn’t afford their brand, then light up when they found the authorized generic. No one’s villainizing the patient here. Everyone’s just trying to survive. This isn’t a loophole-it’s a lifeline with a corporate logo on it.
vishnu priyanka
January 19, 2026 AT 04:08man i just found out my anxiety med has an authorized generic. i was about to switch to a cheaper one but then i remembered last time i did that i felt like i was underwater for a week. now i’m asking my doc for the ‘same pill but cheaper’ version. also i’m lowkey impressed that someone in pharma thought ‘hey, what if we just… not lie to people?’ weird flex but okay.
Clay .Haeber
January 20, 2026 AT 01:57oh wow, so Pfizer is basically a magician who pulls the same rabbit out of the hat twice and calls the second one ‘authorized.’ genius. next they’ll sell the same insulin with a ‘luxury edition’ label and a gold cap. 🤡 you’re not saving me money-you’re just giving me a slightly less shiny version of the same scam. but hey, at least it’s FDA-approved… which means it’s probably also FDA-approved to make you feel guilty for wanting the real thing.
Avneet Singh
January 20, 2026 AT 11:37Authorized generics are a regulatory artifact of the Hatch-Waxman Act’s failure to prevent market monopolization. The NDA-to-ANDA arbitrage is a textbook example of rent-seeking behavior in pharmaceutical economics. The fact that these aren’t listed in the Orange Book demonstrates the structural opacity of FDA’s classification framework. In layman’s terms? It’s a loophole dressed as a solution.
John Pope
January 22, 2026 AT 01:18we’re not talking about medicine here. we’re talking about identity. the pill doesn’t change. the body remembers. the mind panics. the pharmacist says ‘it’s the same’ but you stare at the imprint and think ‘is this still me?’ authorized generics are the existential crisis of pharmacology. we’ve outsourced our trust to a label. and now we’re afraid to trust our own skin.
sam abas
January 23, 2026 AT 22:03so let me get this straight. the company that spent $2 billion developing the drug then turns around and sells the exact same thing for less? what a shocker. next they'll invent a car that drives itself and then sell the same car without the GPS. also why is this even a thing? why not just lower the price of the brand? oh right because then you'd have to admit you were overcharging for 10 years. classic pharma. also i bet the authorized generic has different fillers. i read it on a forum. so it's true.
Alan Lin
January 25, 2026 AT 00:15As a clinician with over 18 years in endocrinology, I’ve seen patients deteriorate on non-authorized generics due to bioavailability inconsistencies. The authorized generic isn’t a marketing ploy-it’s a clinical necessity for patients with narrow therapeutic indices. Thyroid, seizure, and anticoagulant patients are especially vulnerable. Insurance formularies often prioritize cost over clinical stability. That’s not healthcare. That’s actuarial math. I’ve written dozens of prior authorizations for authorized generics because the data is clear: when you match the formulation, outcomes improve. This isn’t about brand loyalty. It’s about physiology.
Angel Tiestos lopez
January 25, 2026 AT 19:00just found out my antidepressant has an authorized generic 😭 i cried. not because i’m sad. because i finally don’t have to choose between my mental health and my rent. also i’m so proud of humanity for making this happen. 🙌🫶 even if it’s still a corporate trick, it’s a *nice* trick. thank you to whoever invented this. you’re a good person. maybe.
Nelly Oruko
January 26, 2026 AT 21:21it’s not about the pill. it’s about the peace.
Adam Vella
January 27, 2026 AT 13:33One must consider the ethical implications of authorized generics within the framework of utilitarianism versus deontological ethics in pharmaceutical distribution. While the immediate utility of cost reduction for the patient is evident, the underlying motive-preservation of market dominance by the innovator-raises questions regarding fiduciary responsibility and the commodification of health. The FDA’s classification system, while legally sound, may inadvertently legitimize a form of regulatory arbitrage that undermines the spirit of generic competition. A true generic market should incentivize innovation in manufacturing, not replication under a different label.