When you’re prescribed a biologic drug-something like Humira for rheumatoid arthritis or Enbrel for psoriasis-you might be shocked by the price tag. These aren’t your everyday pills. They’re complex, lab-grown medicines made from living cells, and they cost thousands per month. But there’s a cheaper option: biosimilars. They’re not generics in the traditional sense, but they’re close. And right now, they’re saving patients and the system billions.
What’s the real price difference?
In early 2025, the average 30-day prescription for a brand-name biologic cost $2,104. The biosimilar version? $919. That’s more than half off. For a patient needing monthly injections for years, that’s tens of thousands saved. Some biosimilars, like those for Humira, launched at discounts of 80% after patents expired. One version, Hyrimoz by Sandoz, now holds 14% of the U.S. market for that drug alone. This isn’t a one-time discount. Once a biosimilar enters the market, the original brand often drops its price too-by an average of 25%. So even if you don’t switch, you still benefit. The result? In 2024 alone, biosimilars saved the U.S. healthcare system $20 billion. Since 2015, total savings have hit $56 billion.Why aren’t biosimilars everywhere yet?
You’d think with savings like this, everyone would switch. But here’s the catch: the system isn’t built for it. Brand drug companies have spent years building what experts call “patent thickets.” They file dozens of minor patents on small changes-like packaging, delivery devices, or dosing schedules-to delay biosimilar entry. Even after the main patent expires, legal battles can push biosimilars back by years. Then there’s the rebate system. Pharmacy Benefit Managers (PBMs), who negotiate drug prices for insurers, often get big kickbacks from brand-name manufacturers. These rebates are tied to how much of the drug gets prescribed. So even if a biosimilar is cheaper, the PBM might still push the more expensive brand because it pays them more. It’s not about what’s best for the patient-it’s about who pays the PBM. Doctors, too, are often unaware of biosimilars or pressured by reps to stick with the brand. Many patients don’t even know they have a cheaper option unless their pharmacist or doctor tells them.Biosimilars aren’t generics-but they’re just as safe
Let’s clear up a common confusion. A regular generic pill, like ibuprofen, is chemically identical to the brand. A biosimilar? It’s not identical. Biologics are made from living cells, so even tiny changes in how they’re grown can alter the final product. That’s why biosimilars are called “highly similar,” not “identical.” But here’s the key: they’re not less effective. The FDA requires biosimilars to prove they work the same way, with the same safety profile, as the original. No clinical trial shows one is better or worse. The FDA approved 76 biosimilars as of October 2025. Every single one met strict standards. Patients who switch from a brand biologic to a biosimilar don’t report more side effects. Studies show no difference in outcomes for conditions like Crohn’s disease, psoriasis, or cancer treatment. The only difference? The price.
How much can you save personally?
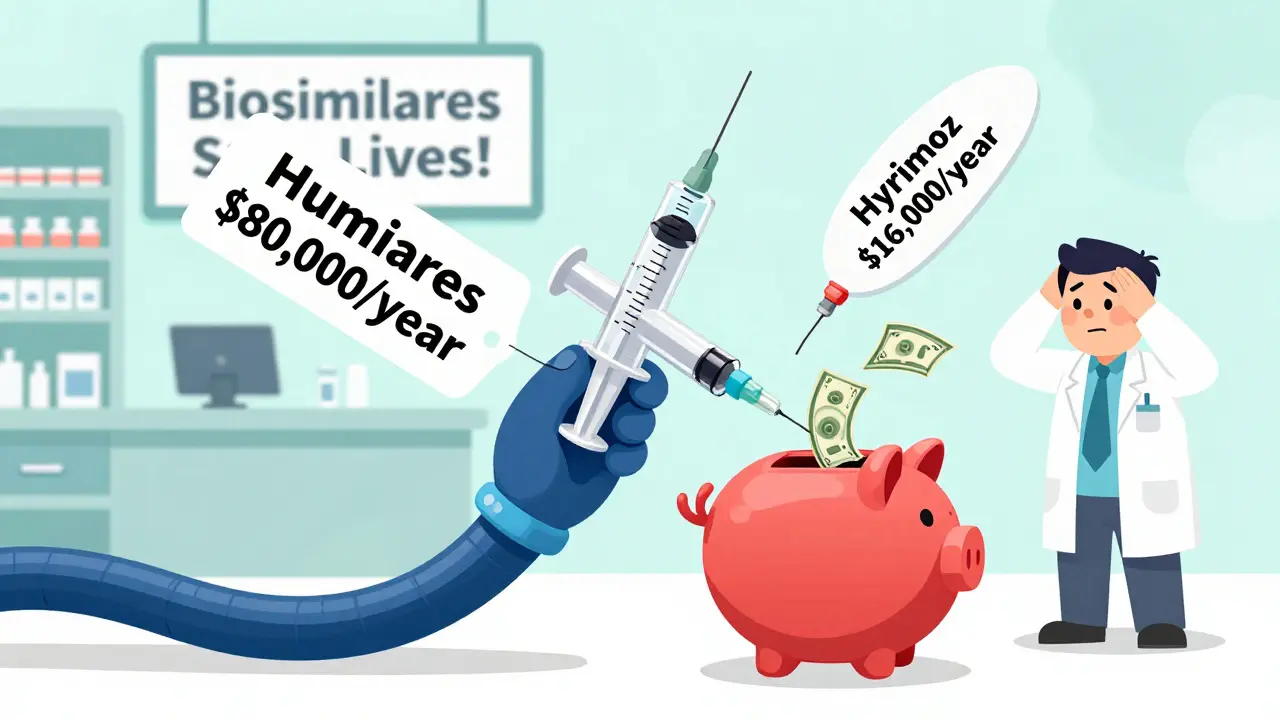
If you’re paying out of pocket, the savings hit hard. For Humira, the brand costs about $80,000 per year. A biosimilar? Around $16,000. That’s $64,000 saved annually. Even with insurance, your copay might still be $500 a month for the brand. With a biosimilar, it could drop to $385. That’s $135 a month, or over $1,600 a year back in your pocket. Some plans have “step therapy” rules-meaning you have to try the cheaper option first. But many don’t. If your prescription is for a biologic, ask your doctor or pharmacist: “Is there a biosimilar available?” If they say no, ask why. It might be a rebate issue, not a medical one.What’s holding biosimilars back?
Despite the savings, biosimilars still make up less than 20% of the biologic market. Compare that to traditional generics, which cover 90% of prescriptions and cost 79% less than brand names. Why the gap? First, development is expensive. Creating one biosimilar costs $100-250 million-far more than a regular generic. That means fewer companies can afford to enter the market. Second, regulatory delays. Even though the FDA is trying to speed things up with new guidance, the process is still slower than it should be. The agency now says it wants to reduce unnecessary clinical trials, which could cut development time by years. Third, policy uncertainty. The Inflation Reduction Act introduced government price controls for some drugs, but it’s unclear how that affects biosimilars. Companies are hesitant to invest if they don’t know what prices they’ll be allowed to charge later.
The future: More savings on the horizon
The good news? Things are changing. The Biden administration launched a Biosimilars Action Plan in late 2025 to tackle patent thickets and rebate walls. The FDA is streamlining approval. Health plans are starting to shift incentives-some now cover biosimilars at zero cost to patients. Analysts predict biosimilar market share will jump from 15-20% today to 35-40% by 2030. That could mean over $125 billion in annual savings. For patients, this isn’t just about money. It’s about access. Right now, many people skip doses or skip treatment entirely because they can’t afford the brand. Biosimilars could change that. A patient with an autoimmune disease might go from choosing between rent and medication to living a full, active life.What you can do today
If you or someone you know is on a biologic drug:- Ask your doctor: “Is there a biosimilar version?”
- Ask your pharmacist: “Can I switch?”
- Call your insurance: “Do you cover biosimilars? What’s my copay?”
- Don’t assume your prescription is the only option-ask for alternatives.
Why this matters beyond your wallet
Biologics are the fastest-growing part of drug spending. They make up only 5% of prescriptions but 51% of total drug costs. If we don’t fix this, premiums, taxes, and out-of-pocket costs will keep rising for everyone. Biosimilars are the most powerful tool we have to bring those costs down. They’re not perfect. They’re not magic. But they’re real. And they’re working. The question isn’t whether biosimilars are safe or effective. It’s whether we’re willing to use them.Are biosimilars the same as generics?
No. Generics are exact chemical copies of small-molecule drugs, like aspirin or metformin. Biosimilars are highly similar versions of complex biologic drugs made from living cells. They’re not identical, but they work the same way and have the same safety profile as the original brand.
Are biosimilars safe?
Yes. The FDA requires biosimilars to prove they’re as safe and effective as the brand-name drug. Thousands of patients have switched, and studies show no increase in side effects or loss of effectiveness. The FDA has approved 76 biosimilars as of October 2025, all meeting strict standards.
How much cheaper are biosimilars?
On average, biosimilars cost 40-50% less than the brand at launch. For some drugs like Humira, discounts reached 80%. Out-of-pocket costs for patients are 23% lower with biosimilars. In 2024, biosimilars saved the U.S. system $20 billion.
Why don’t doctors prescribe biosimilars more often?
Many doctors aren’t trained on biosimilars or are influenced by pharmaceutical reps pushing the brand. Insurance rebates also make it financially easier for PBMs to favor the more expensive drug. Patients often need to ask for a biosimilar-don’t wait for your doctor to bring it up.
Can I switch from a brand biologic to a biosimilar?
Yes, and it’s often safe. The FDA and major medical groups support switching. Many patients do it without issues. Check with your doctor and pharmacist first, but don’t assume you’re locked into the brand. Ask if switching is an option.
Will my insurance cover a biosimilar?
Most do, but coverage varies. Some plans require prior authorization or step therapy. Call your insurer and ask: “Do you cover biosimilars for [drug name]? What’s my copay?” If they say no, ask why-it might be a rebate issue, not a medical one.
 Jan, 15 2026
Jan, 15 2026
Rob Deneke
January 16, 2026 AT 16:52Man I switched my mom to the Humira biosimilar last year and her copay dropped from $520 to $370. She’s been on it for 10 months now and no issues. Her rheumatoid arthritis is actually better controlled. I wish more people knew this was an option.
evelyn wellding
January 18, 2026 AT 10:09OMG YES!!! 💪 I literally cried when my pharmacist told me my Enbrel biosimilar was $150 a month instead of $700. My insurance didn’t even make me jump through hoops. Just switched and boom-saved $6,600 a year. Thank you for posting this!! 🙌
Chelsea Harton
January 19, 2026 AT 14:18patent thickets are just corporate greed dressed up as law. why do we let them do this? it’s not innovation it’s obstruction.
Corey Chrisinger
January 20, 2026 AT 05:06It’s wild how we’ve normalized paying $80k for a drug when the science behind it isn’t even that new. Biosimilars are like the open-source movement of pharma-same function, less cost. But the system’s built to protect profits, not people. We’re not broken-we’re just misaligned.
Bianca Leonhardt
January 20, 2026 AT 06:05People still don’t get it? You’re literally leaving money on the table and risking your health by sticking with the brand. If you’re not asking for biosimilars, you’re being manipulated. Stop being passive. Your life isn’t a corporate spreadsheet.
Travis Craw
January 21, 2026 AT 21:01my doctor never mentioned biosimilars until i asked. i felt kinda dumb for not knowing sooner. but now i know. and i’m telling everyone i know. it’s not hard to ask. just do it.
Christina Bilotti
January 22, 2026 AT 15:33Oh wow, a post that actually doesn’t sound like a pharmaceutical ad? Shocking. And let me guess-you think patients are just too lazy to ask? No, sweetheart, they’re too busy working three jobs to read 2000-word essays on drug pricing. Maybe fix the system before you lecture people on ‘asking questions’.
brooke wright
January 24, 2026 AT 04:37Wait so if I’m on a biosimilar and my insurance says no coverage, is that because of rebates or because they’re just being jerks? I’ve been denied twice and I’m tired of playing phone tag with my PBM. Can someone explain what I’m supposed to say when they say ‘it’s not covered’?
vivek kumar
January 25, 2026 AT 19:23India’s biosimilar market is growing fast because we had no choice. We couldn’t afford Western prices. Now we export biosimilars globally. The U.S. has the tech, the capital, the infrastructure-but not the will. It’s not about science. It’s about power.
Nick Cole
January 26, 2026 AT 05:01My sister has Crohn’s and switched to a biosimilar last year. She went from missing work every other week to running marathons. I don’t care what the reps say or what the rebate says-this isn’t theoretical. This is real life. If you’re not advocating for this, you’re part of the problem.
Riya Katyal
January 27, 2026 AT 18:01Wow, you wrote a whole novel on biosimilars and didn’t mention how the FDA approval process is still way slower than it should be? Like, come on. You say ‘streamlined’ but the average time is still 5 years. That’s not progress, that’s a delay tactic dressed in bureaucracy.
Henry Ip
January 28, 2026 AT 18:14Just talked to my pharmacist today. He said if your doctor says ‘no biosimilar,’ ask them to check the PBM’s formulary. Most times it’s not medical-it’s just the rebate structure. If you push, they’ll change it. I’ve seen it happen. Don’t give up.