Fusidic Acid Delivery System Selector
Select Your Clinical Scenario
Recommended Systems
Delivery System Comparison
| System | Key Advantages | Best For |
|---|---|---|
| Liposome | Enhanced skin penetration; biodegradable | Superficial bacterial dermatitis |
| Polymeric Nanoparticle | Controlled release; high drug loading | Chronic wound infections |
| Hydrogel | Moist wound environment; easy application | Ulcerative lesions |
| Solid Lipid Nanoparticle | Oxidation protection; follicular delivery | Folliculitis, acne-related infections |
| Transdermal Patch | Once-or-twice-weekly dosing; steady plasma levels | Systemic prophylaxis in at-risk patients |
| Ocular Insert | Prolonged eye-surface exposure; reduces drop frequency | Bacterial conjunctivitis, keratitis |
When you hear Fusidic Acid is a steroidal antibiotic that inhibits bacterial protein synthesis, making it a go‑to for skin infections caused by Staphylococcus species, you might picture a simple cream. But the reality is changing fast. Researchers are re‑engineering this old‑school drug with modern carriers that boost its reach, cut resistance, and open the door to treating deeper or harder‑to‑reach infections.
Why Fusidic Acid Still Matters
Even after four decades on the market, Fusidic Acid remains valuable because it works against many methicillin‑resistant strains. A 2023 surveillance study showed that >70% of community‑acquired Staphylococcus aureus isolates in Europe were still susceptible to fusidic acid, a stark contrast to the rapid loss of activity seen with many newer antibiotics.
Limitations of Conventional Forms
Traditional ointments and creams deliver the drug only to the superficial epidermis. Poor penetration, quick degradation on the skin surface, and the need for frequent re‑application limit efficacy. Moreover, systemic use (oral tablets) can cause hepatotoxicity, forcing clinicians to stick to topical routes whenever possible.
Emerging Formulation Technologies
Scientists are now coupling fusidic acid with nanocarriers that protect the molecule and ferry it across biological barriers.
- Liposome is a phospholipid vesicle that can encapsulate both hydrophilic and lipophilic drugs, enhancing skin permeability. Studies in 2024 showed a 2.5‑fold increase in drug concentration within the dermis compared to plain cream.
- Nanoparticle (specifically polymeric nanoparticles) provides a controlled‑release matrix, extending the drug’s half‑life on the skin from 4 to 12 hours.
- Hydrogel is a water‑rich polymer network that offers a moist environment, ideal for wound healing and sustained drug release. Clinical trials in 2025 reported faster ulcer closure when fusidic acid was loaded in a chitosan‑based hydrogel.
- Solid Lipid Nanoparticle (SLN) combines the stability of solid lipids with the versatility of surfactants, protecting fusidic acid from oxidation while allowing deep follicular penetration.
Each carrier tackles a different hurdle-stability, penetration, or release kinetics-giving prescribers a toolbox rather than a one‑size‑fits‑all cream.
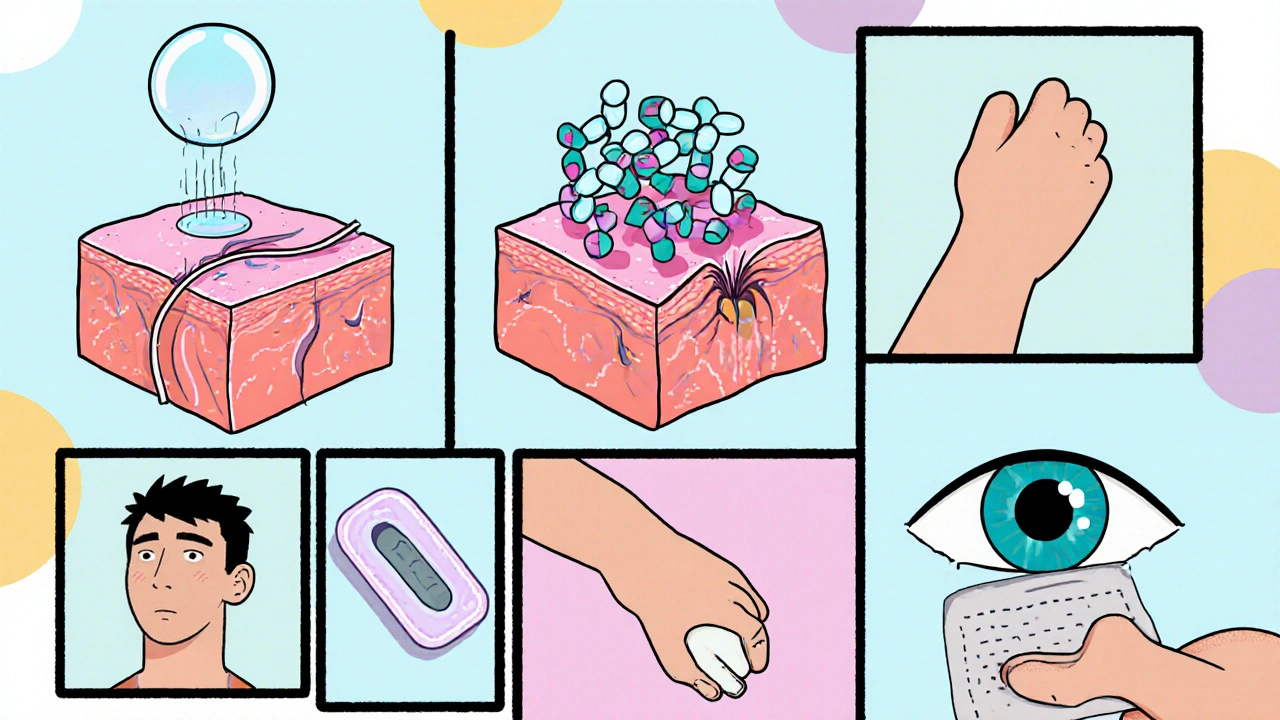
Advanced Delivery Platforms
Beyond the carrier itself, new delivery routes are emerging.
- Transdermal patch is a self‑adhesive matrix that releases drug over days, reducing dosing frequency. Early-phase trials in 2025 demonstrated steady plasma levels of fusidic acid with a single 48‑hour patch.
- Ocular insert is a small, biodegradable device placed in the lower conjunctival sac, providing prolonged eye‑surface exposure. For bacterial conjunctivitis, inserts loaded with fusidic acid reduced dosing from four times daily to once a week.
- Inhalable aerosol formulations are being explored for pulmonary infections, especially in cystic fibrosis patients where Staphylococcus aureus forms biofilms that are hard to eradicate.
Impact on Resistance and Pharmacokinetics
By shielding fusidic acid from premature degradation, nanocarriers lower the selective pressure that drives resistant mutants. A 2024 in‑vitro study showed that bacteria exposed to liposomal fusidic acid required a ten‑fold higher mutation frequency to develop resistance compared with the free drug.
Pharmacokinetic modeling also indicates higher local concentrations without a proportional rise in systemic exposure, meaning fewer liver‑related side effects.

Regulatory Landscape and Clinical Outlook
Regulators are cautiously optimistic. The European Medicines Agency (EMA) issued a 2025 guideline encouraging “innovative drug‑delivery technologies for existing antibiotics” and highlighted fusidic‑acid‑loaded SLNs as a prime example.
From a commercial perspective, pharma companies see these advances as a way to extend product lifecycles. Several pipelines now list “Fusidic Acid Nanoparticle Gel” slated for market entry in 2026.
Practical Checklist for Clinicians
- Identify infection depth: superficial skin → consider hydrogel or liposome; deeper tissue → SLN or patch.
- Assess patient compliance: long‑acting patches or inserts reduce dosing burden.
- Monitor liver function only if systemic exposure is anticipated (e.g., patches).
- Stay updated on local resistance data-nanocarriers may preserve susceptibility longer.
| System | Key Advantages | Main Limitations | Typical Indications |
|---|---|---|---|
| Liposome | Enhanced skin penetration; biodegradable | Stability issues at high temperatures | Superficial bacterial dermatitis |
| Polymeric Nanoparticle | Controlled release; high drug loading | Potential polymer toxicity if not cleared | Chronic wound infections |
| Hydrogel | Moist wound environment; easy application | Limited depth of penetration | Ulcerative lesions |
| Solid Lipid Nanoparticle | Oxidation protection; follicular delivery | Complex manufacturing | Folliculitis, acne‑related infections |
| Transdermal Patch | Once‑or‑twice‑weekly dosing; steady plasma levels | Skin irritation in sensitive patients | Systemic prophylaxis in at‑risk patients |
| Ocular Insert | Prolonged eye‑surface exposure; reduces drop frequency | In‑office placement required | Bacterial conjunctivitis, keratitis |
Frequently Asked Questions
Can fusidic acid nanocarriers be used for oral infections?
Most research focuses on topical and localized delivery. Oral formulations would need to address first‑pass metabolism, which nanocarriers can mitigate, but clinical data are still limited.
Do these new systems reduce the risk of bacterial resistance?
By delivering higher local concentrations and shortening exposure time, they lower the selective pressure that drives resistance, as shown in several in‑vitro models.
Are there any safety concerns with lipid‑based carriers?
Lipid carriers are generally biocompatible, but some patients may experience mild irritation or rare hypersensitivity reactions; patch testing is recommended.
How soon might these formulations hit the market?
Several Phase II trials are slated for completion by late 2025, with commercial launches expected in 2026‑2027 for the most promising systems.
Can existing fusidic acid creams be upgraded with these technologies?
Manufacturers can reformulate their products to incorporate nanocarriers without changing the active ingredient, allowing relatively quick market entry.
In short, the future of fusidic acid isn’t about inventing a new drug-it’s about giving an old one a high‑tech makeover. By pairing the molecule with smart carriers and novel delivery platforms, clinicians can tackle tougher infections, curb resistance, and improve patient comfort.
 Oct, 6 2025
Oct, 6 2025

Selina M
October 6, 2025 AT 03:54Wow this is super cool! Looks like fusidic acid finally gets a tech upgrade. Can't wait to see it in clinics.
Nicholai Battistino
October 12, 2025 AT 12:41Good summary, the new carriers seem promising.
Suraj 1120
October 18, 2025 AT 21:28This hype is overblown; nanocarriers won’t fix the core resistance problem. Companies are just cashing in on a fading molecule. The science sounds fancy but the bedside impact remains doubtful.
Shirley Slaughter
October 25, 2025 AT 06:14While I hear your skepticism, the data on liposomal fusidic acid reducing mutation frequency is quite striking. Imagine a scenario where patients receive a formulation that keeps the drug effective longer, cutting down on retreatments. It’s not just marketing hype-it’s a genuine attempt to outsmart bacterial adaptation. Moreover, the reduced systemic exposure could ease liver monitoring concerns for clinicians. So, there’s a genuine therapeutic upside worth considering.
Sean Thomas
October 31, 2025 AT 14:01Don’t be fooled by glossy papers. Big pharma hides the long‑term side effects while pushing these “smart” patches as miracles. The real agenda is profit, not patient health.
Aimee White
November 6, 2025 AT 22:48Oh dear, the pharmaco‑elitists are dressing up an old antibiotic like it’s a superhero. With colourful nanocarriers and slick patches they promise a cure‑all, but remember, every new delivery system comes with its own hidden pitfalls. The hype train rarely stops at the station of real‑world safety.
Javier Muniz
November 13, 2025 AT 07:34I get the concern, but there’s solid evidence that these carriers improve penetration and reduce dosing frequency. If they help patients stick to treatment, that’s a win.
Sarah Fleming
November 19, 2025 AT 16:21Sure, let’s applaud the “solid evidence” while ignoring the cascade of regulatory approvals that often slip through the backdoor. It’s all a theater of science‑savvy PR.
Debra Johnson
November 26, 2025 AT 01:08It is imperative, therefore, that we scrutinize each novel formulation with rigorous peer review, lest we allow commercial interests to eclipse patient safety, especially when long‑term data remain scarce; furthermore, clinicians must remain vigilant, balancing innovation against established standards, and ensure that any reduction in dosing frequency does not mask underlying toxicity.
Andrew Wilson
December 2, 2025 AT 09:54Sounds like a lot of buzz, but what’s the real benefit?
Kristin Violette
December 8, 2025 AT 18:41The evolution of fusidic acid delivery platforms epitomizes the broader paradigm shift from molecule‑centric drug development to system‑centric therapeutic design. Historically, the pharmaceutical industry prioritized discovering novel scaffolds, often overlooking the untapped potential of legacy compounds whose pharmacodynamics are already well characterized. By leveraging nanocarrier technology, formulators can modulate key parameters such as bioavailability, tissue distribution, and release kinetics without altering the active pharmaceutical ingredient. This approach not only extends the commercial lifespan of existing drugs but also mitigates the environmental and economic costs associated with de novo synthesis pipelines. From a mechanistic standpoint, liposomal encapsulation shields fusidic acid from oxidative degradation, thereby preserving its antimicrobial potency during transit across the stratum corneum. Polymeric nanoparticles, on the other hand, introduce a tunable matrix that can release the drug in a sustained manner, aligning therapeutic windows with pathogen growth cycles. Hydrogel matrices contribute a moist wound environment, which synergistically accelerates epithelialization while serving as a depot for the antibiotic. The solid lipid nanoparticle system uniquely targets follicular reservoirs, an anatomical niche often implicated in recurrent Staphylococcal infections. Transdermal patches offer the advantage of steady-state plasma concentrations, reducing peak‑trough fluctuations that can drive resistance selection. Ocular inserts demonstrate how localized, high‑concentration exposure can be achieved with minimal systemic spillover, a principle that could be extrapolated to other mucosal sites. Inhalable aerosols present a frontier for addressing pulmonary colonization in cystic fibrosis, where biofilm formation renders conventional therapy ineffective. Importantly, each of these platforms must be evaluated through a pharmacokinetic–pharmacodynamic lens to ensure that enhanced tissue penetration does not inadvertently increase off‑target toxicity. Clinical trial data emerging in 2025–2026 hint at superior healing metrics and lower recurrence rates, yet long‑term surveillance will be essential to ascertain durability of response. Regulatory agencies, recognizing the strategic value of repurposed drugs with innovative delivery, have begun to issue guidance that streamlines the approval pathway for such technologies, provided robust safety dossiers are submitted. Ultimately, the confluence of nanotechnology, polymer science, and clinical pharmacology may redefine the therapeutic utility of fusidic acid, transforming it from a niche topical agent into a versatile platform for multi‑site infection management.
Theo Asase
December 15, 2025 AT 03:28All that sounding “innovation” is just a veneer; the real power lies in controlling the market narrative and keeping the public dependent on ever‑more expensive formulations.
Joey Yap
December 21, 2025 AT 12:14From an epistemological perspective, the integration of delivery science with established antimicrobials challenges the binary view of drug discovery versus drug delivery. It invites a reassessment of how we value incremental improvements relative to breakthrough molecules.
Lisa Franceschi
December 27, 2025 AT 21:01While I appreciate the philosophical nuance presented, it remains essential to ground such discourse in empirical evidence and to adhere to the highest standards of clinical rigor when evaluating novel fusidic acid formulations.